One of the most basic experiments taught to everyone in school, the titration between an acid and a base helps us to calculate the concentration of a solution, whose volume is known. This ScienceStruck article provides you with a step-by-step procedure of this experiment along with proper inferences.
Derived from?
The word “titration” descends from the Latin word titulus, which means inscription or title.
Titration is simply defined as the procedure wherein an acid reacts with a base, whose volumes are known and concentrations are unknown. Using the known values, the concentration of the compound (analyte or titer) can be calculated by reacting or neutralizing it with another chemical compound called titrant. An indicator solution is used to determine the endpoint of the reaction between both these solutions. In this experiment involving a reaction between sodium hydroxide (titrant) and sulfuric acid (titer), an indicator called phenolphthalein is used. It is colorless in acids, and its endpoint is marked by a color change to pink, when the entire volume of the analyte has reacted with a small amount of the titrant.
The molecular formula of sulfuric acid is H2SO4. It is a highly corrosive acid made from sulfur dioxide, and is known to be among the most extensively used products in the chemical industry. Sodium hydroxide (NaOH) is also an important base that is used in factories, which is involved in the manufacture of cleaning products, water purification techniques, and paper products. This compound is a strong alkali, and is also known as lye and/or caustic soda. The following paragraphs will explain the entire titration procedure in a classic chemistry experiment format.
Aim
- To calculate the concentration or molarity of sulfuric acid solution by reaction with a basic solution of sodium hydroxide.
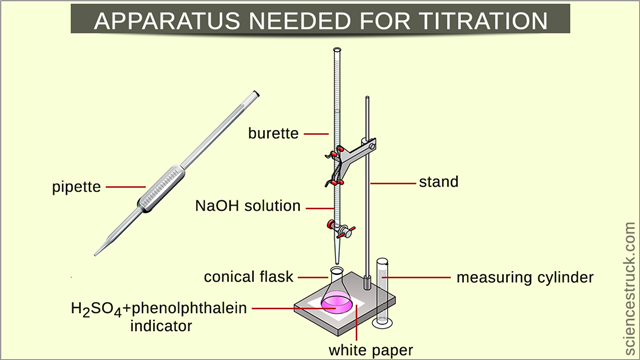
Apparatus/Equipment Needed
- Burette (a measuring instrument consisting of a graduated glass tube with a tap at the bottom) with a stopcock at the bottom .
- 10 ml pipette (a measuring instrument consisting of a graduated glass tube used to measure or transfer precise volumes of a chemical by drawing the liquid up into the tube) with a round bulb at the top portion .
- Titration stand to fix the burette.
- Three 250 ml conical glass flasks .
- One 100 ml glass beaker .
- An indicator dye (a chemical that shows a different color in an acid and a base); in this experiment, it’s recommended to use phenolphthalein, 50 ml dilute H2SO4 solution in a beaker, 500 ml NaOH solution, 25 ml measuring cylinder, and white paper.
Procedure
1. Firstly, clean the burette with distilled water (DW), and then rinse it with sodium hydroxide (NaOH) solution. This ensures that the interior portion of the burette is coated with a thin layer of NaOH solution.
2. Clean the pipette with DW, and rinse it with dilute solution of H2SO4. This is done by carefully drawing about 10 ml of this solution into the pipette, and releasing it into the sink. Do not let it enter your mouth or touch any part of your body.
3. Now, fix the burette on the stand, and fill it with NaOH solution just above the zero mL mark. By turning on the stopcock, release some of the solution in a clean 100 ml flask to remove any air bubbles in the burette. Refill it up to the zero mL mark, such that the lower meniscus of the solution is touching just above this mark.
4. Draw 10 ml dilute H2SO4 by a pipette, and release it into a 250 ml flask.
5. Add 2 – 3 drops of phenolphthalein indicator to the acid, and keep the flask just below the burette stopcock on a white paper.
6. Now, hold the flask just below the burette tip, and slowly turn the stopcock so as to release the NaOH solution in a drop-by-drop manner inside the flask. Simultaneously, keep swirling the flask in a gentle way as sodium hydroxide starts reacting with the acid.
7. When a slight pink color can be seen in the mixture against the white paper, stop adding the base by turning off the stopcock.
8. Swirl 2 or 3 more times to see if the pink color disappears. If it remains, then the reaction is complete, and if the mixture again turns colorless, then start adding the base slowly till a color change is noticed.
9. Note the reading of the volume used by NaOH to react with the acidic solution. This is done by observing the position of the lower meniscus in the burette.
10. Repeat this procedure at least 3 times by using 3 different flasks, and note down the readings of NaOH used every time. Take the average of all three readings for calculating the concentration of sulfuric acid.
Process, Observations, and Reactions
1. The indicator used, i.e., phenolphthalein has an endpoint from colorless to pink. This means that it is colorless in acids and turns pink in bases. Thus, as it is added in the flask at the start of the procedure, the acidic solution remains colorless. When a specific volume of NaOH has reacted with the acid, the color change is observed; it is very crucial to stop the addition of the titrant at this point, as any extra amount of the base can give a wrong reading, thus, leading to entirely wrong calculations. At this point, the reaction being complete, the indicator detects the presence of adequate amount of a base solution in the mixture and hence, turns pink.
2. A reaction between sulfuric acid and sodium hydroxide is of an acid-base type, or is also known as a neutralization reaction. In this process, both compounds undergo a reaction to neutralize the acid and base properties. The products of this process are salt and water. The former is produced when the cation (a positively charged ion) of the base combines with the anion (a negatively charged ion) of the acid. In the language of chemistry, the cation of the base NaOH, i.e., Na+, combines with the anion of the acid H2SO4, i.e., SO42–. This gives out Na2SO4. Water is formed as a result of the reaction between the cation of the acid (H+) that combines with an anion of the base (OH–).
The balanced equation of this reaction is:
H2SO4 + 2NaOH → Na2SO4 + 2H2O —- (equation 1)
3. In this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (NaOH) combine with one mole of sulfuric acid (H2SO4). This results in the formation of two moles of water (H2O) and one mole of sodium sulfate (Na2SO4). The net ionic equation (a chemical equation in which electrolytes are written as dissociated ions) can be explained as follows:
The complete balanced equation for the reaction between sulfuric acid and sodium hydroxide is:
H2SO4 + 2NaOH → Na2SO4 + 2H2O
Thus, writing this in a full ionic reaction form , we get:
2H+ + SO42- + 2 Na+ + 2OH– → 2Na+ + SO42- + 2H2O
So, the net ionic equation of sulfuric acid and sodium hydroxide, after crossing out the spectator ions, is:
2H+ + 2OH– → 2H2O
Calculations and Inferences
Let’s find out the molarity or concentration of sulfuric acid solution from the given and observed data (molarity and observed volume of sodium hydroxide).
For example, during three rounds of the experiment, the amount of NaOH needed to react with sulfuric acid is 12 ml, 13 ml, and 12.5 ml, respectively. Thus, by taking their average, 12.5 ml of NaOH neutralized the acid with the unknown concentration. Suppose the molarity of sodium hydroxide is 0.1 mol/L. The formula for calculating the number of moles of a solution is:
Number of Moles (N) = Volume (V) x Molarity (M)
Thus, the formula becomes,
N = 0.0125 x 0.1 = 0.00125 moles
Now, according to the equation 1 mentioned above, exactly half the number of moles of sulfuric acid take part in the reaction; i.e., the number of moles required for the neutralization process are:
N (for sulfuric acid) = 0.00125/2 = 0.000625 moles
The volume of sulfuric acid used in this experiment is 10 ml. By rearranging the above formula, the molarity or concentration of H2SO4 is calculated:
Molarity (M) = Number of Moles (N)/Volume (V) M = 0.000625/0.01 = 0.0625mol/L
Thus, the molarity or concentration of sulfuric acid in the above-described experiment is 0.0625 mol/L.
Titration experiments are not only useful for understanding neutralization reactions between acids and basis, but also help us understand reactions related to stoichiometry, industrial applications, groundwater analysis, studies related to hard water and detergents, etc. The endpoint is not always characterized by a color change; in some experiments, it can also be indicated by ways like precipitation and variation in the electrical conductivity of the reactants.