How is ionization potential defined? Why does it affect the reactivity of any atom? Read to find the answers.
All of theoretical chemistry, related to atomic and molecular reactions, is quantum physics, which studies the energy changes associated with electrons and provides a system for probability calculations of atomic and molecular orbitals. An important concept associated with atomic physics is the ionization potential of an atom.
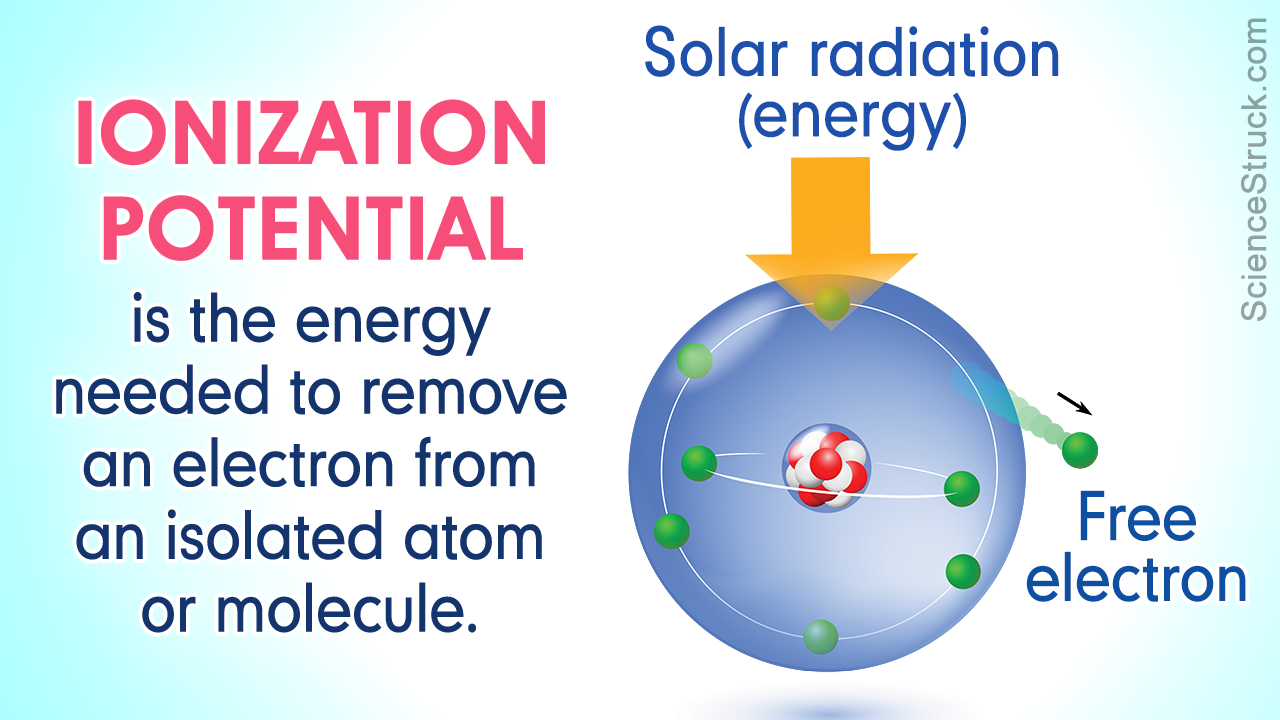
An atom consists of a central nucleus containing positively charged protons and neutrons, which is surrounded by electrons revolving around it in orbitals with specific energy levels. The electrons are bound to the atomic nucleus, because of the electromagnetic force of attraction.
To break free from the attractive force of the atomic nucleus, an electron requires energy which may be supplied from external sources. For every atom of a chemical element, the energy levels for each atomic orbital are different.
Definition
Also known as ionization energy, ionization potential is defined as the total amount of energy required to free an electron, which is revolving in the outermost shell of any atom. Alternatively, chemists also define it as the total energy required to liberate one mole of electrons, from one mole of a particular atom.
In physics, we prefer using electron volts (eV) per atom, as a unit for ionization potential, while in chemistry, it’s measured in terms of kJ/mol (Kilojoules per mole). For example, the ionization energy of hydrogen is 13.5984 eV. The lower the energy, higher is the tendency of an atom to become a reducing agent and more reactive it is. On the other hand, higher the ionization energy, higher is the tendency to become an oxidizing agent and less reactive it is.
Equation
To be able to measure the ionization potential for any atom, one must solve the Schrodinger equation from quantum physics, to calculate the energy levels of that atom. A complete analytical solution for any atom, other than Hydrogen is not possible. An estimate of the energy can be made on the basis of an approximate model. Here is the required equation:
Ionization Potential (eV) = 13.6 eV x (Z2/n2)
where Z is the atomic number of the atom and n is the orbital number. This formula can only provide you with an approximate value. A more precise value can only be obtained from experiments.
The energy required to liberate one electron from an atom’s outer energy shell is known as first ionization potential, while the energy required to liberate from the second one is known as the second ionization potential. Second ionization potentials are always higher than first ones. That is because, more you take away the electrons from its outermost shells, more tightly an atom hold the rest of the electrons, which are left.
As we saw before, the key point to understand is that higher the ionization energy of an atom, greater will be the energy required to remove an electron from inner level orbitals of the atom. In conclusion, remember that atoms with low ionization energy, lose electrons easily and are hence, good reducing agents. On the other hand, atoms with high energy are more likely to be oxidizing agents. As an exercise, take a look at the ionization potential table for all elements and guess which elements are likely to be reducing or oxidizing agents.