Cations and anions are positively and negatively charged atoms, respectively. ScienceStruck elaborates on the differences between the two, through a cation vs. anion comparison.
Did You Know?
The words anion and cation are derived from the Greek words ano, meaning up, and kata, meaning down, respectively.
An atom is the smallest unit of matter and is invisible to the naked eye. It is composed of three distinct types of minute subatomic particles – protons, neutrons, and electrons. Protons and neutrons occupy the central part of the atom, called the ‘nucleus’, whereas, the electrons float around the nucleus in various orbits. Protons carry a positive charge, while electrons carry a negative charge. However, neutrons carry no electrical charge at all.
An atom is said to be in a stable state when it comprises equal number of protons and electrons that neutralize the electric charge. However, there is a possibility that an atom or group of atoms may gain or lose electrons, making it either positively or negatively charged. Such atoms are referred to as ‘ions’. Depending upon the charge it acquires, an ion is classified into two types – cation and anion.
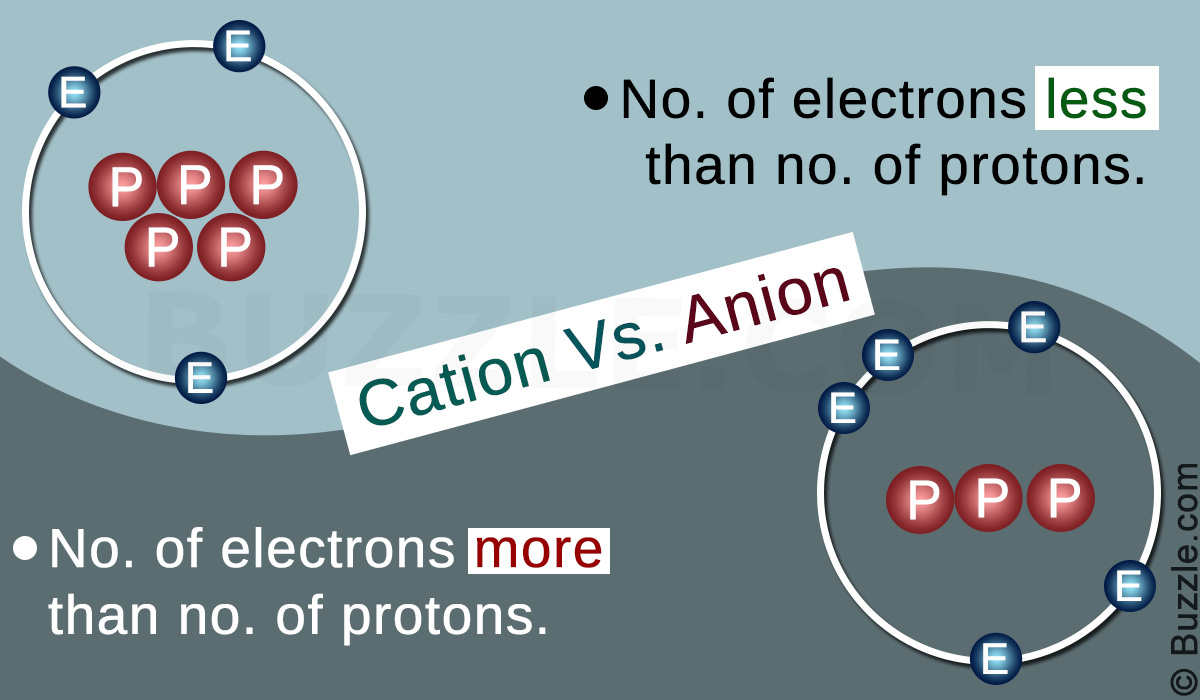
CATION
- A cation is an atom which has lost one or more electrons. This gives a net positive charge to the cation, since the protons that are positively charged outnumber the electrons.
- A cation is smaller in size than its respective atom, since the loss of an electron decreases the repulsive power of the electrons. This in turn enables the protons to attract the electrons towards the nucleus, thereby reducing the radius of the cation.
- It is represented using the ‘+’ sign. For instance, the cation Na+ indicates that it has one electron less than the protons. Similarly, a cation with +4 charge has four electrons less than the total number of protons.
- Cations get attracted towards the cathode (negative electrode) during electrolysis.
- They are usually formed by metals through endothermic reactions.
- Example of the formation of a hydrogen cation from a hydrogen atom:
H – e– ➞ H+
ANION
- An anion is an atom which has gained one or more electrons. This gives a net negative charge to the anion, since the electrons that are negatively charged are high in number as compared to the protons.
- An anion is larger than its respective atom, since the addition of electrons in the outermost orbit increases the electron-electron repulsion, which pushes the electrons further apart, thereby increasing the radius of the anion.
- It is represented using the ‘-‘ sign. For instance, the anion Cl- indicates that it has one electron more than the protons. Similarly, an anion with -2 charge has two electrons more than the total number of protons.
- It gets attracted towards the anode (positive electrode) during electrolysis.
- Anions are usually formed by non-metals through exothermic reactions.
- Example of the formation of an iodide anion from an iodine atom:
I + e– ➞ I–
The following table lists the cations and anions.
Cation and Anion Chart
| CATIONS (+ve) | |
| Name | Formula |
| Ammonium | NH4+ |
| Cesium | Cs+ |
| Copper(I) (Cuprous) | Cu+ |
| Hydrogen | H+ |
| Hydronium | H3O+ |
| Lithium | Li+ |
| Potassium | K+ |
| Silver | Ag+ |
| Sodium | Na+ |
| Barium | Ba2+ |
| Beryllium | Be2+ |
| Calcium | Ca2+ |
| Chromium(II) (Chromous) | Cr2+ |
| Copper(II) (Cupric) | Cu2+ |
| Iron(II) (Ferrous) | Fe2+ |
| Lead(II) | Pb2+ |
| Magnesium | Mg2+ |
| Manganese(II) (Manganous) | Mn2+ |
| Mercury(I) (Mercurous) | Hg22+ |
| Mercury(II) (Mercuric) | Hg2+ |
| Nitronium | NO2+ |
| Sodium | Na+ |
| Strontium | Sr2+ |
| Tin(II) (Stannous) | Sn2+ |
| Zinc | Zn2+ |
| Aluminum | Al3+ |
| Chromium(III) (Chromic) | Cr3+ |
| Iron(III) (Ferric) | Fe3+ |
| Manganese(III) (Manganic) | Mn3+ |
| Tin(IV) (Stannic) | Sn4+ |
| ANIONS (-ve) | |
| Name | Formula |
| Acetate | CH3COO– |
| Amide | NH2– |
| Bromate | BrO3– |
| Bromide | Br– |
| Chlorate | ClO3– |
| Chloride | Cl– |
| Chlorite | ClO2– |
| Cyanate | OCN– |
| Cyanide | CN– |
| Dihydrogen phosphate | H2PO4– |
| Fluoride | F– |
| Formate | HCOO– |
| Hydride | H– |
| Hydrogen carbonate | HCO3– |
| Hydrogen sulfate | HSO4– |
| Hypobromite | OBr– |
| Hypochlorite | OCl– |
| Hydroxide | OH– |
| Iodate | IO3– |
| Iodide | I– |
| Nitrate | NO3– |
| Perchlorate | ClO4– |
| Permanganate | MnO4– |
| Thiocyanate | SCN– |
| Carbonate | CO32- |
| Chromate | CrO42- |
| Dichromate | Cr2O72- |
| Hydrogen phosphate | HPO42- |
| Oxalate | C2O42- |
| Oxide | O2- |
| Peroxide | O22- |
| Sulfate | SO42- |
| Sulfide | S2- |
| Sulfite | SO32- |
| Thiosulfate | S2O32- |
| Arsenate | AsO43- |
| Arsenite | AsO33- |
| Nitride | N3- |
| Phosphate | PO43- |
Source: Arkansas State University, Department of Chemistry and Physics
Anions and cations together form neutral ionic compounds in which the number of positive charges are equal to the number of negative charges. For instance, sodium chloride (NaCl) or table salt is formed when sodium and chlorine ions bond together. The sodium atom loses an electron to become Na+, while the chlorine atom gains an electron to become Cl-. Since the number of electrons lost is equal to the number of electrons gained, the ionic compounds do not have a net electric charge.