Scandium is a transition metal element, which was discovered in 1879. It has since found various applications in different fields. Find out more about its uses, and also some interesting facts about this metal, in this article.
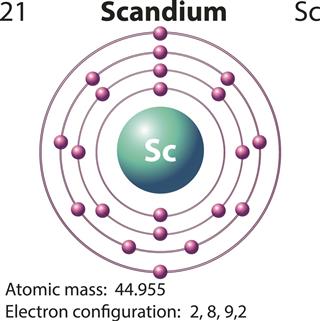
Scandium belongs to group IIIB of the periodic table. In chemistry, it is represented by the symbol ‘Sc’. It was discovered by Swedish chemist, Lars Fredrik Nilson, in the year of 1879. Nilson and his team detected scandium when they were working with the rare minerals Euxenite and Gadolinite, which were brought from Scandinavia. Hence, the chemical element derived its name from the Latin word ‘Scanda’, which means Scandinavia.
Facts
Scandium is a light metal, and it has a soft and smooth texture. Here are some interesting facts about this element:
- In its pure form, it has a silvery-white color. On exposure to air, the silvery-white color of this metal gets a yellowish or a pinkish tinge.
- Its uniqueness lies in the fact that it has a low density, but its melting point and boiling point are quite high. Its melting point is 1,541 degrees Celsius, and its boiling point is 2,830 degrees Celsius.
- The atomic number of scandium is 21. Like any other element from group IIIB of the periodic table, it has a valency of 3. In other words, during a chemical reaction, when it combines with other elements, it loses three electrons from in its outermost shell (or valence shell).
- In all, there are eleven recognized isotopes of scandium. Among them, naturally occurring scandium-45, is a stable isotope. The other ten are radioactive isotopes with unstable nuclei.
- As far as its chemical properties are concerned, it is quite reactive in nature. When it reacts with water, it produces hydrogen. It readily reacts with a number of acids. At room temperature, it does not react with the oxygen present in the air, but when ignited, it burns readily in the air.
Important Uses
The use of scandium started only in the 1970s,(;) this was mainly due to the fact that, it was not readily available. No, it is not a rarely found element on the Earth. In fact, there are more than 800 minerals in which scandium can be found,(;) however, in most of these minerals, it is found only in trace amounts. Moreover, it is always found in a combined state. As a result, extraction of this metal was a major problem.
One of its most important uses is for preparing aluminum-scandium alloy, which is used in the aerospace industry during the manufacturing of aircraft. When added in a trace amount (about 0.1% to 0.5%) to aluminum, it increases the strength of aluminum manifold, without increasing its weight.
Its use in aerospace industry though, is restricted to specialized aircraft (like the Russian military aircraft MiG-21, MiG-29, etc.), owing to the high cost of this element. Another key use of this alloy is in the manufacturing of various sports equipment, like baseball bats, lacrosse sticks, and bikes. All these items have a common requirement: a high performance material, which is light in weight, rust resistant, and which has a high melting point. Aluminum-scandium alloy satisfies all of these requirements.
Commercially, scandium is used on a large scale for making metal halide lamps. These lamps are actually partially modified mercury vapor lamps in which a combination of scandium iodide and sodium iodide is added, so as to get an effect of high intensity white light. The artificial light thus produced, is quite similar to natural sunlight. These lamps are in such great demand(,) that it is estimated that almost 80 kg of scandium is used every year all over the world for the production of these lamps.
Well-known American gun-making company, Smith & Wesson, uses scandium alloy for making the frames of its revolvers. Scandium-46, which is a radioactive isotope of the element, is used in oil refineries, where it plays the role of a tracing agent. The compound scandium triflate acts as a catalyst in organic chemistry. Scandium can also be used for the purpose of polishing glass.
Scandium, in its elemental form, is non-toxic. However, studies have found that some of its compounds could be carcinogenic in nature. Therefore, it must be handled with care, and any kind of exposure must be avoided.