Krypton is one of the six rare or noble gases. Find out about the different physical and chemical properties of krypton in this article.
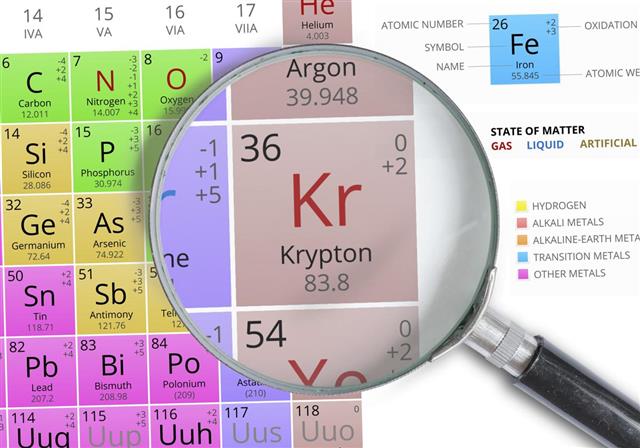
A noble gas is an element that is essentially in gaseous state and which has all completely filled orbits in its atom. Krypton is one of the six noble gases. (The other noble gases are Helium, Neon, Argon, Xenon and Radon). The symbol of krypton is Kr. The atomic number (or charge number) of krypton is 36 – it means krypton has 36 protons in its nucleus and 36 electrons orbiting the nucleus.
Sir William Ramsay (whose sketch/painting you can see in the picture alongside) discovered the element krypton in the year 1898, much more than a century ago. He was awarded the Nobel Prize in Chemistry in the year 1904 for the discovery of krypton and other noble gases. Krypton is present in infinitesimally small quantities in nature – it is present at a concentration of 1 ppm in air. 1 ppm stands for 1 part per million; means for a million parts of air, there will be only one part of krypton! Since its discovery, krypton has found many uses.
Physical Properties of Krypton
| Property | For Krypton |
| 1. Physical state : It refers to one of the three common states (gaseous, liquid and solid) of matter that exist at NTP (Normal Temperature and Pressure). | Krypton is an element in gaseous state at NTP. |
| 2. Melting point : It is the temperature at which a substance changes from solid to liquid state. | Melting point of krypton is -157.36°C. |
| 3. Boiling point : It is the temperature at which a substance changes from liquid to gaseous state. | Boiling point of krypton is -153.22°C. |
| 4. Triple point : It is the temperature and pressure at which all the three states of matter of a substance coexist in perfect thermodynamic equilibrium (i.e. the substance simultaneously exists in all three states of matter, viz. solid, liquid and gaseous.) | Triple point of krypton is -157°C. |
| 5. Crystal structure : It is the unique pattern in which atoms (or molecules) of a crystalline substance are arranged. | Krypton exhibits a face-centered cubic crystal lattice. |
| 6. Magnetism : It refers to the property of materials to respond to an externally applied magnetic field. | Krypton is diamagnetic (i.e. it is repelled by an external magnetic field). |
| 7. Miscellaneous properties | Colorless, odorless, tasteless |
Chemical Properties of Krypton
| Property | For Krypton |
| 1. Isotopes : An isotope is a form of an element that has the same atomic number (or charge number) but different atomic weight, owing to varying numbers of neutrons present in the nucleus of an atom. | Krypton has 6 stable isotopes. |
| 2. Electronegativity : It is the amount of energy required to add an electron to the outermost orbit of an atom (i.e. ability of an atom to attract electrons). | Electronegativity of krypton on the Pauling scale is 3.00 |
| 3. Ionization potential (IP) : It is the energy required to remove an electron from the outermost orbit of an atom (i.e. ability of an atom to give an electron). | 1st IP (energy to remove 1st electron) of krypton is 1350.8 kJ·mol−1. 2nd IP (energy to remove 2nd electron) of krypton is 2350.4 kJ·mol−1. |
| 4. Oxidation state : It indicates the degree of oxidation of the atom of an element. | Krypton has two oxidation states. |
| 5. Chemical nature : It describes the general chemistry of the element. | Krypton is an unreactive gas. Hence it is also called an inert or a noble gas. |
In spite of being a chemically unreactive element, krypton has been used to synthesize many compounds that find applications in making neon lamps, flash light for high-speed photography, etc. There are many uses of krypton, even if it is a rare gas at 1 ppm in the atmosphere. Probably this is something we should learn from the gas – though inconspicuous, it is mighty useful!