The first animal enzyme to be discovered and crystallized, pepsin is a proteolytic enzyme that breaks down proteins into peptides. This ScienceStruck post provides information on the structure, function, and important facts about pepsin.
Did You Know?
Pepsin was discovered by German physiologist Theodor Schwann, in 1836. In 1930, it was isolated in the crystalline form by John H. Northrop of the Rockefeller Institute for Medical Research.
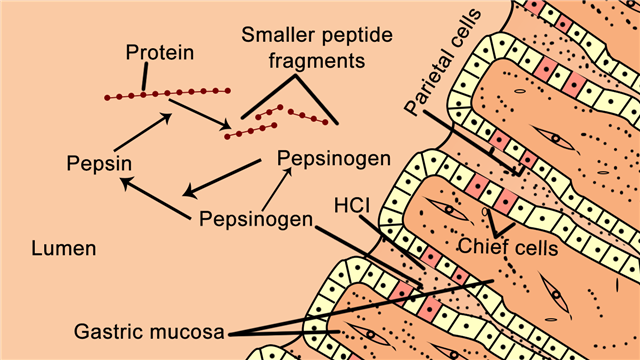
The lining of the human stomach has millions of gastric glands. Following each meal, these glands perform the vital function of secreting gastric juice. Different types of cells are found within these glands. These include the parietal cells, chief cells, mucus-secreting cells, and hormone-secreting cells.
The parietal cells secrete hydrochloric acid and intrinsic factor, whereas the mucus-secreting cells secrete mucin. The chief cells produce and secrete pepsinogen, which is the precursor to the proteolytic enzyme pepsin.
It must be noted that the hormone-secreting cells in the stomach stimulate the gastric glands to secrete gastric juice. After the ingestion of food and its arrival in the stomach, the vagus nerve sends signals that stimulate the release of gastrin in the bloodstream. Once it comes in contact with the gastric glands in the stomach, it triggers the secretion of gastric juice, which comprises hydrochloric acid, mucus, and digestive enzymes such as rennin and pepsin.
Pepsin Enzyme Function and Uses

Pepsin, chymotrypsin, and trypsin are placed under the category of proteolytic enzymes. These enzymes are involved in the hydrolysis of proteins into peptides and amino acids by breaking them down into peptide bonds.
Pepsin is quite effective in breaking down peptide bonds in case of amino acids such as phenylalanine, tryptophan, and tyrosine. Pepsin is secreted in the form of pepsinogen, which is a zymogen (proenzyme or an inactive precursor). It is the release of hydrochloric acid by the parietal cells in the stomach lining that causes the inactive precursor pepsinogen to change into the active form of pepsin. Hydrochloric acid helps maintain the optimum acidity (pH 1-3) for pepsin function.
In the stomach, the pepsinogen molecules digest one another partially, thereby removing segments of polypeptide chains, and converting pepsinogen into pepsin. Pepsin breaks the peptide bonds between the amino acids with the hydrophobic side chains in the middle of polypeptides. Thus, it changes long polypeptides into short polypeptides.
The process of conversion of proteins into peptones can be achieved with the help of commercially-prepared pepsin. One of the pepsin applications is in the food industry, wherein the enzyme is used for curdling milk in cheese-making. It might also be used for processing soy protein and gelatin, so as to impart them a whipping quality. It might be used for the preparation of vegetable and animal protein hydrolysates that can be used as flavoring agents in food or beverages. In food chemistry, pepsin can be used for assessing the digestibility of proteins. Pepsin was historically an additive of Beemans gum brand chewing gum by Dr. Edward E. Beeman. In the leather industry, it is used for removing the tissue and hair remnants from the hides and softening them. It is also used in the laboratory analysis of various proteins.
Pepsin Enzyme Structure
Basically, proteins are large polymers that are joined by peptide bonds. The peptide bond is an amide linkage that joins the amino group of one amino acid to the carboxyl group of another. Proteins comprise several types of amino acids, with the difference being in the chemical nature of the side chain. When many amino acids are joined in this manner, it leads to the formation of a peptide. Polypeptides are formed when hundreds of amino acids are joined in this manner. In case of proteins, one or more polypeptide chains are held together by non-covalent interactions. They have an N-terminus, where the amino group of the end amino acid is unlinked, and a C-terminus, where the carboxyl group of the end amino acid is unlinked.

Pepsinogen has an additional 44 amino acids on its N-terminus. During the transformation of pepsinogen into pepsin, these 44 amino acids are released. While pepsin has fewer basic amino acid residues, it has 44 acidic residues. This is the reason why it remains stable at extremely low pH. To prevent self-digestion, pepsins need to be stored at very low temperatures that range between −80°C and −20°C.
Pepsin hydrolyzes the peptide bonds of proteins, breaking them down into smaller polypeptide fragments. It is active under the acidic conditions that are provided by the presence of gastric juice in the stomach. The low pH of the stomach is due to the secretion of HCl by the gastric glands. The strong acidity of the stomach denatures the proteins from ingested food, thereby increasing the exposure of the peptide bonds of protein. Pepsin works best at the acidity of normal gastric juice, which has the pH that ranges between 1.5 and 2.5. It must be noted that the reaction for the conversion of pepsinogen into pepsin requires a pH value of less than 5. Pepsin is not effective in the intestine, as the gastric acids are neutralized, with the pH value being 7. Laboratory studies have revealed that pepsin is most efficient in cleaving bonds involving the aromatic amino acids that include phenylalanine, tryptophan, and tyrosine.
On a concluding note, the impulses from the vagus nerve, as well as the secretion of gastrin and secretin hormones stimulate the release of pepsinogen into the stomach, where it is mixed with hydrochloric acid and quickly converted to the pepsin enzyme. It must be noted that pepsin is only involved in the partial degradation of proteins. The main site of protein digestion is the intestine, wherein the trypsin, chymotrypsin (secreted by the pancreas), and others work on the digestion of proteins, thereby breaking them down into peptides, which in turn are converted into amino acids.