Distillation is the standard process used for separation of chemical mixtures. In this article, a comparison between fractional and simple distillation has been presented, which will identify the prime differences between the two processes.
If you are planning to major in chemistry, be ready to spend long hours in the laboratory. It’s a hands on science, which involves the study of chemical properties and their reactions. Among the many laboratory techniques, which form a necessary part of a chemist’s training, one of the most important ones is the process of distillation. Two primary types of distillation processes are fractional and simple distillation. Both techniques are used in industries and research departments devoted to production and analysis of chemicals.
What is Distillation?
Right at the outset, let me explain what is distillation, before we get into the comparison of two of its specific types. A mixture is an assortment of two or more substances. Most substances in nature occur in the form of a mixture and there are an array of techniques in chemistry to separate these mixtures into their component parts. Distillation is a process which separates the components of a mixture with different boiling points, through application of heat.
It’s a process which has been in use since ancient times and continues to be used even today in modern refineries and chemical laboratories. It is mostly used to separate components from liquid solutions. Purification of water through distillation is an example. Depending on the nature and boiling points of the components, different distillation techniques are employed.
What is Simple Distillation?
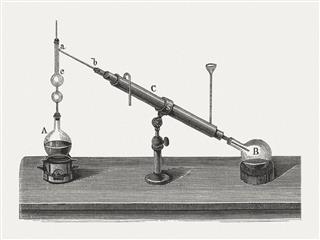
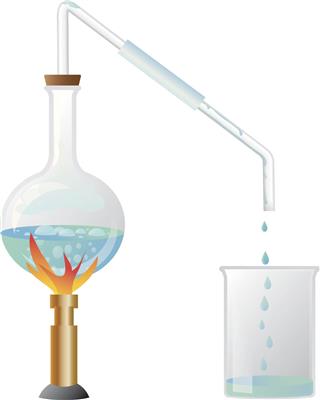
This technique is generally used to separate substances in a mixture, whose boiling points differ largely. It is generally used in the separation of volatile components of the mixture from the other non-volatile components with higher boiling points. In a simple distillation process, the mixture is placed in a flask and heated. As the temperature rises and reaches a boiling point threshold, the volatile component separates out in vapor form and passes through the long neck of a retort (a flask with a long tapering glass snout) to condense at its bottom, back to liquid form. Thus, the volatile component is separated out from the mixture. If there are more than one volatile components in the mixture, they are all bound to get separated in vapor form simultaneously, making the process useless. Hence, it’s important that this technique should only be used when boiling temperature of components is greater than 20 to 25°C.
What is Fractional Distillation?
This is a process which is used to separate out components with boiling points that are very close to each other. The apparatus for fractional distillation consists of a fractionating column, through which the vaporized components pass and go through repeated cycles of condensation and vaporization, until all the components have separated out.
The column consists of a series of ladder like glass plates where the various components, depending on their boiling points, condense at different heights, after going through repetitive cycles of vaporization. The most volatile substance with the lowest boiling point reaches the top of the column and then passes through a condenser to regain liquid form. Thus, fractionally, each mixture component is separated out. This is the most widely used distillation method used in petroleum refineries and chemical factories to separate out various chemicals from their mixtures.
Difference Between Simple and Fractional Distillation
When the substances in a mixture have boiling points which differ largely, simple distillation is the first choice. When that isn’t the case, fractional distillation is used. This process uses a fractionating column. Simple distillation cannot guarantee high purity of the separated component but fractional distillation can provide separated chemicals with a high degree of purity.
Based on the same principle, the two techniques differ in procedure. To summarize the differences, fractional distillation is used in separating mixtures, whose components have boiling points that are too close to each other, while simple distillation is used in case of mixtures whose components have boiling points that are substantially far apart. Of the two types, fractional distillation is more widely used in industries and laboratories.