In chemistry, there are three basic types of reactions, which include addition reactions, decomposition reactions, and displacement reactions. Among them, this ScienceStruck article comes forward to discuss in length about a type of displacement reaction called single replacement reaction, accompanied by various examples.
| Did You Know? To protect structures made of iron from corroding, a more reactive metal like zinc is added to it. It reacts with the environment and corrodes itself, while the iron is protected. Such metals are aptly given the name “sacrificial metal.” |
In chemistry, the compounds that take part in a chemical reaction are called reactants, whereas the compounds that are formed after a chemical reaction are called products. Reactants are physically and chemically distinct from the products that they make. A chemical reaction can be given as:
A + B ➝ C + D
As a rule, the reactants A and B are written on the left-hand side, and their products are written on the right-hand side of the arrow (signifies a chemical reaction).
In a displacement reaction, there is displacement of one of the reactants by another reactant. Usually, these reactions take place between a metal and non-metal that are joined by an ionic bond. Displacement reactions can be further classified into single replacement reaction and double replacement reaction. In single replacement reactions, one ion or an atom of an element is displaced by another ion or atom (discussed in detail below). In a double replacement reaction, there is interchanging of ions or atoms between the reactants.
Single replacement reactions or single displacement reactions involve the replacement of an atom or an ion from one compound by a more reactive compound. This type of a reaction can be depicted in the following manner:
▶ Here, A is a more reactive compound as compared to B; B is, therefore, displaced from the compound B-C to give the products B and A-C. A and B are usually metals (give off electrons) or a positively charged hydrogen. C is usually a non-metal or an anion (accepts electrons) and usually considered as a spectator ion as it doesn’t take part in the reaction.
▶ How are some compounds more reactive than others?
Reactivity of an element (usually metals) can be determined by the ease with which it gives off an electron. The order their reactivity can be given as follows:
Note: Hydrogen is given here for comparison.
▶ In our surroundings, we encounter some examples of single replacement reaction.
In the extraction of iron from its ore, ferric oxide is heated with carbon. Carbon displaces iron at very high temperature, and then elemental iron is formed.
▶ There are two types of single replacement reactions:
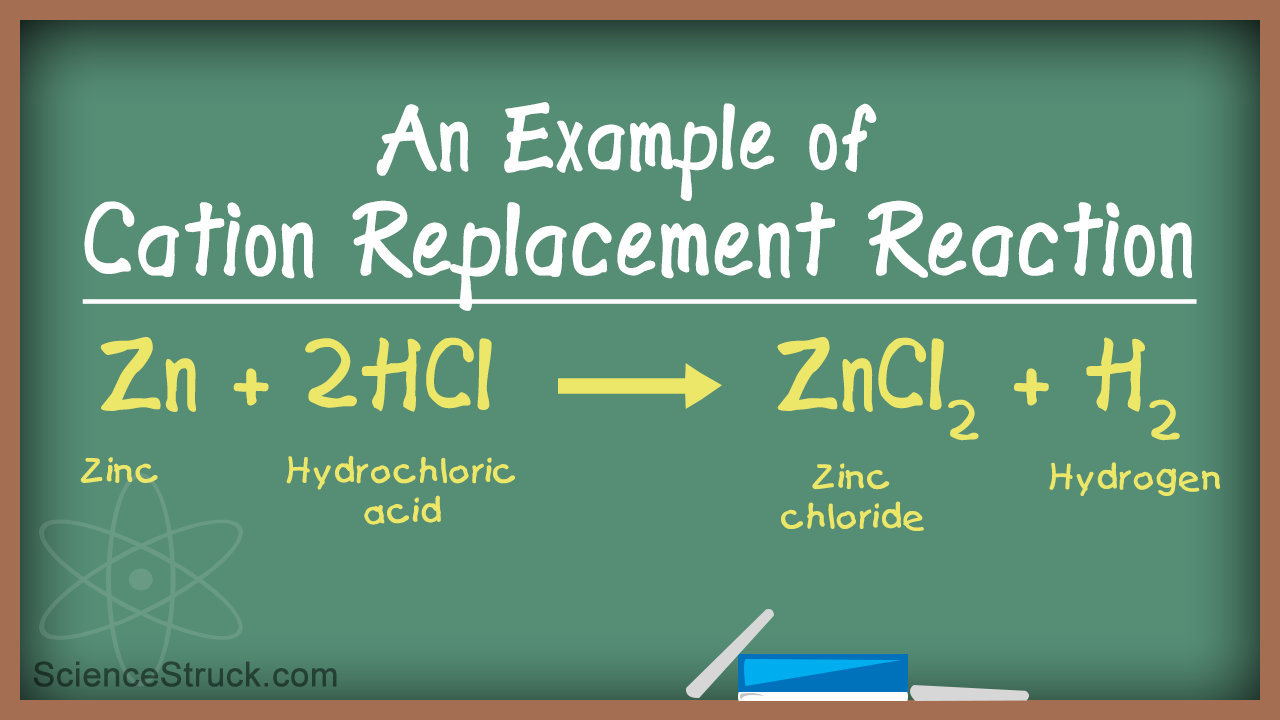
In this type of reaction, a cation (positively charged ion) replaces another cation that is present in the compound.
Here, one cation X+ replaces another cation Y+.
These reactions are considered as redox reactions as there is simultaneous reduction of one reactant, and there is oxidation of the other reactant. X+takes up electrons and undergoes reduction, whereas Y+ gives off its electron and undergoes oxidation reaction.
Breaking down this reaction, we get;
Zn+Cl + HCl + H0 ➝ ZnCl2 + H2(g)
Breaking down this reaction, we get;
Ca+(OH) + H0 + H2O ➝ Ca(OH)2 + H2
Breaking down this reaction, we get;
Zn+NO3 + Ag0 + AgNO3 ➝ Zn(NO3)2+ 2Ag0
In this type of reaction, an anion (negatively charged ion) replaces another anion that is present in the compound.
Here, one anion P– replaces another anion R–.
These reactions are also considered as redox reactions as there is a simultaneous reduction of one reactant and oxidation of the other. P takes up electrons and undergoes reduction, whereas R– gives off its electron and undergoes an oxidation reaction.
Halogens are highly electronegative elements. These elements take up electrons rather easily, and the ease with which they take up electrons refers to the reactivity of these elements. The reactivity of halogens is given below:
Breaking down this reaction, we get;
NaCl + Br– + Cl0 + NaBr ➝ 2NaCl + Br2
Breaking down this reaction, we get;
NaBr + Br– + I0 + NaI ➝ 2NaBr+ I2
Breaking down this reaction, we get;
KBr + Br– + I0 + KI ➝ 2KBr+ I2
Both anion and cation replacement reactions are irreversible reactions, where the more reactive ion or atom replaces the less reactive one.