The entirety of our organic world is created through covalent bonding of atoms. Through examples, we explain the nature of this chemical bond.
If you observe the world at a microscopic level, you will realize that its complexity arises from clumping together of simpler units of matter, called atoms. The electromagnetic force binds atoms together to form molecular compounds, which aggregate together to create even bigger molecules and polymers, which make life possible.
This ascending order of complexity is made possible by the electromagnetic forces that exist between charged particles. Chemical bonding at the level of atoms, through sharing or exchange of electrons makes the creation of molecules possible. Bonding between atoms can either be covalent or ionic.
What is a Covalent Bond?
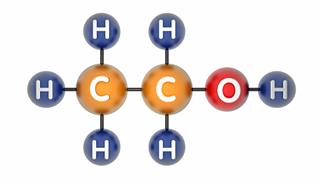
A covalent bond between atoms is formed, when they share one or more pairs of electrons among each other. These shared pairs create a bond between the atoms, which binds them together into a singular unit, as a molecule. These shared electrons are the valence electrons of atoms, which revolve in their outermost shells.
Each atom, that forms a covalent bond, tries to achieve stability, by filling up the outermost electronic orbitals of atoms, which are still unoccupied. As the two atoms come together and share electrons, a bond is created by the electromagnetic force of attraction which exists between the atomic nuclei and the shared pair of electrons.
There is also a force of repulsion between the electrons revolving around both atoms, which causes the molecule to get distorted. Since both atomic nuclei are attracted to the shared pair of electrons, a bond is created between the two atoms.
Depending on the electron affinity or electronegativity (tendency of atoms to attract electrons), the shared pair of electrons may be equally or unequally shared. If they are unequally shared, the molecule becomes ‘polar’, in the sense that imbalance of charges is created within.
Examples
Hydrogen Chloride
Hydrogen Chloride (HCl) is another polar molecule, where the electron pair is pulled more towards the chlorine atom, which has a higher electronegativity.
Water
In Water(H2O) molecule, two hydrogen atoms share their single electrons with the oxygen atom, which shares its own two electrons in return. This is an example of a polar covalent bond, which is created because of the higher electronegativity of oxygen.
Hydrogen
Hydrogen Molecule (H2) is a non-polar covalent bond example, as an electron pair is equally shared between the two hydrogen atoms.
Ammonium Chloride
Ammonium Chloride (NH4Cl) is a coordinate covalent bond example, where both electrons required for bonding, are supplied by the same atom.
Here is a table listing molecules with polar and non-polar bonds.
| Polar Covalent Bond | Non-Polar Covalent Bond |
| Hydrogen Fluoride (HF) | Nitrogen (N2) |
| Ozone (O3) | Methane (CH4) |
| Ammonia (NH3) | Carbon Dioxide (CO2) |
| Hydrogen Sulfide (H2S) | Chlorine (Cl2) |
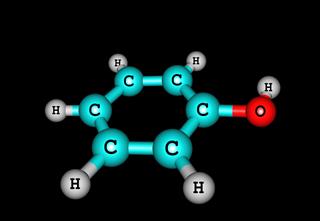
These were some illustrative examples, which should have given you an idea about the nature of this type of chemical bond. As discussed before, the sharing of electrons between the atoms, which constitute the molecule, is influenced by their individual electronegativity. The more unequal the electronegativity, more polar is the molecule formed. More matching the electronegativity, more equally shared the electrons are. Almost all organic molecules, made up of carbon chains, are covalently bonded.
Chemistry is devoted to the study of a wide range of molecules, ranging from the simplest, like those of oxygen, to the more complex molecules like DNA (Deoxyribonucleic Acid). Each of these molecules are held together by covalent bonding, which exists between its constituent atoms. Study more examples in greater detail, to understand molecular bonding more thoroughly.