The following article provides information regarding the bromine test; the method of conducting it and its uses.
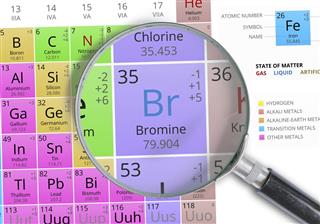
Bromine belongs to the halogen group of chemical elements in the periodic table. Its atomic number is 35, and it possesses an atomic mass of 79.904. Although it is a relatively rare element in the Earth’s crust, the fact that it is highly soluble causes bromine ions to be present in oceans and naturally occurring saline water bodies. Owing to this reason, it can be easily extracted from saltwater pools and other brine water sources for commercial utility. Appearance-wise, elemental bromine exists as a dense, translucent, mobile, and reddish-brown liquid, which readily transforms into a reddish-brown vapor when exposed to standard temperature and pressure.
Although a nonmetallic element, bromine may assume metallic properties when a 55 GPa pressure is applied to it. As a chemical, it reacts actively with metals. In case water is also present at the time of such a reaction, bromine salts are produced. This element has the ability to easily form bonds with many other elements. It also possesses bleaching properties.
Bromine Test
It is used to detect the presence of unsaturated compounds of alkene and alkyne. Such a test for alkenes work via the mechanism of making alkenes or hydrocarbons, having a minimum of one double bond that undergoes addition reactions. The alkenes and hydrocarbons combine with bromine to impart a colorless appearance to this element. The equation for this reaction is represented as follows:
H2 = CH2 —> H2BrC – CbrH2
An addition reaction is the phenomenon, where a smaller molecule adds on across a double bond. Since alkenes contain double bonds, they are capable of undergoing addition reactions. As for the change in bromine’s color, alkenes are colorless and therefore, their combination with bromine causes the latter to lose color, as well as it gets consumed in the reaction process. A bromine test is also conducted to detect the presence of phenolic compounds like phenol and cresol. A bromine water test is one of the best, easiest, and surest ways to detect the presence of unsaturated double bond compounds and phenols in any unknown sample. The presence of phenols, however, can be identified differently from the presence of alkenes and unsaturated double bonds, as when phenols come in contact with bromine solutions, the resultant precipitate is white in color.
Bromine Test Strips
Basically, they are commercially available strips of bromine coated material, which are used to test the alkalinity, hardness, and pH of swimming pool and spa waters. These tests are essential to detect undesirable chemical elements in water, in order to maintain water quality. This is true especially in case of beauty and wellness spas, which run therapeutic baths. Any imbalance in the chemical composition of such bath water may be detrimental to the client’s wellness and beauty regime. Some well-known bromine test strip brands, which market the strips to test swimming pool and spa water for alkalinity and hardness are as follows:
- Leisure Time Spa Bromine Test Strips
- Advantis Technologies Leisuretime Bromine Test Strips
- Aquacheck 3 Way Bromine Test Strips
- Aquacheck Red “bromine” Pool Spa Test Strips
- Tru-Blu Sodium Bromide Test Strips
Most common instances of a bromine test includes its performance in laboratories, as part of chemistry practicals, and for testing water quality of swimming pools and spas.