Transition metals are extremely versatile elements, which show potential for application in various fields such as medicine, construction, energy, etc., due to their properties of malleability and ductility. Let us now study the uses of these unique elements in some more detail.
| Did You Know? Of all the elements in the periodic table, only the transition metals cobalt, nickel, and iron are capable of producing a magnetic field of their own.
|
The transition metals consist of 38 elements in the periodic table situated in the groups 3-12. Transition metals are both ductile and malleable, and usually lustrous in appearance. They also are good conductors of heat and electricity. These elements are well-known for their various oxidation states, which is possible due to the presence of the valence electrons (electrons that form compounds by joining with other elements) in more than one shell.
Scandium, cadmium, titanium, hafnium, vanadium, tantalum, chromium, tungsten, manganese, rhenium, iron, osmium, cobalt, iridium, nickel, platinum, copper, gold, zinc, mercury, yttrium, rutherfordium, zirconium, dubnium, niobium, seaborgium, molybdenum, bohrium, technetium, hassium, ruthenium, meitnerium, rhodium, ununnilium, palladium, ununbium, unununium, and silver, are all transition metals. The unique properties of these elements make them essential in the creation of many items that serve our everyday needs right from creating the humble iron nail to the construction of spacecraft. We will now look at the uses of some transition metals, and learn why they are important for the functioning of a civilized society.
Applications of Transition Metals
Iron
This is the most abundantly available transition metal, and 4th most abundant element of the periodic table. Iron is very useful, especially when mixed with other elements to prepare alloys such as steel. Such alloys have many industrial uses such as the manufacture of construction and building materials, tools, vehicles, cosmetics, paints, fertilizers, etc., making it extremely important for the world economy. Cast iron is usually used to make covers for manholes. Iron is also used as a catalyst, while artificially extracting ammonia in the Haber process. Most importantly, iron is a very important element in the bodies of many animals, used to create hemoglobin which is used to carry oxygen in the blood.
Scandium
Scandium forms a 25 parts per million component of the Earth’s crust. The element is usually extracted from a rare mineral named thortveitite, or while processing uranium from various ores. It is used in the television industry, because it has a good light output, that is similar to the solar spectrum. Some isotopes of scandium are used to analyze crude oil samples. Aluminum alloys are strengthened by a big margin when trace amounts of scandium are added to them.
Vanadium
Vanadium is another abundantly available element that is found in over 60 minerals. It is mainly found as a byproduct during the production of uranium and crude oil. Vanadium is important in creating steel alloys that are stronger and more resistant to wear and tear, that are primarily used to create high speed tools. It is used sometimes to bond steel and titanium. This element is also used in the chemical industry for the production of sulfuric acid.
Manganese
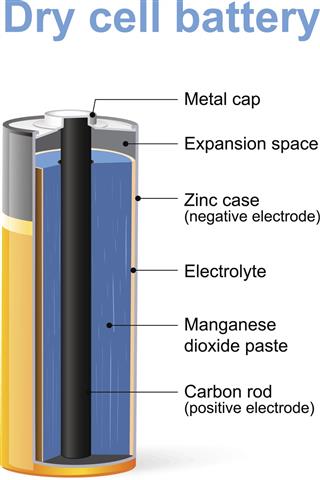
Manganese is the 3rd most abundant transition metal available. Most of this metal is found on the floors of the oceans around the world. Majority of the manganese that is extracted goes into the production of ferro-manganese steel. The remainder goes into creating components of electronic devices, owing to its low temperature coefficient of resistivity. It is also used to create potassium permanganate, which is used in chemical labs across the world. Manganese is also used in dry batteries, the manufacture of bricks and glass, fertilizers, and the production of magnets that are used in televisions.
Cobalt
This element is quite rare, and is primarily used in the ceramics and paint industry to create blue colors. Some compounds of cobalt are used in the creation of aldehydes that are useful in the chemical industry. It is also used to make stellite, an alloy used for making drill bits. This element is also used to produce high temperature-resistant alloys that are used in the manufacture of gas turbines. Magnetic alloys such as alnico steel are also manufactured with the help of cobalt.
Nickel
A large chunk of the world’s production of nickel comes from a massive meteor crater in Sudbury Canada, indicating that the minerals in that area originated from space. It is primarily used to produce ferrous and non-ferrous alloys, such as alnico steel and stainless steel. It is also used in the manufacturing process of vegetable oil and margarine. It also has several uses in chemical labs and industries across the world.
Copper
A very important element in terms of electrical systems that use copper wires, it is also used in making currency coins in many countries. It is used to make lightning rods, heating and cooling systems, etc. Besides this, it is a major part of alloys such as brass, bronze, and monel. Also, because it is so malleable and non-reactive with water, it is used to manufacture water pipes, heaters, and cooking dishes.
Zinc
Over 6 million tons of zinc is produced annually, majority of which is used to coat various metals to prevent corrosion and rust. It is also used in the manufacture of batteries and in the process of die casting. It is also an important component of brass alloys. Zinc oxide is used in manufacturing textiles, paints, and rubber products. Zinc sulfide is used in televisions, clocks, and fluorescent light bulbs. The element is also a part of rat poisons. The human body uses zinc to heal wounds and to store insulin inside the pancreas.
Silver
Silver is a good conductor of electricity, and it does not corrode in air or water. This makes it ideal for manufacturing jewelry, printed circuit boards, and electric contacts, especially when alloyed with copper. It is a very important element in the photograph developing process, and is found in cadmium batteries.
Gold
Everyone knows, that gold is a high value metal. Similar to silver, gold does not corrode, and it is a great conductor of electricity. It is used for making computer chips, wires, electric contacts, and jewelry. Some radioactive isotopes of gold are also used as implants to cure certain cases of cancer.
Platinum
Initially, most miners considered platinum to be an annoyance that regularly came in their way while they searched for gold. Today, it is usually more expensive and more useful. It is primarily used to make jewelry due to its properties of durability, tarnish resistance, and beautiful color. Used in manufacturing fuel cells and nitric acid, it is also used as a catalyst in automobiles to reduce the polluting emissions of carbon monoxide and nitrogen oxides. It is also sometimes used in manufacturing thermometers and rocket engines due to its ability to withstand high temperatures. Platinum can also be found in pharmaceutics and medical fields for manufacturing anti-cancer drugs and surgical equipment.
Cadmium
Even though cadmium is a metal, it is soft enough to be easily cut with a knife. It is a silvery white element that is used in electroplating metals to protect them from corrosion and rust. It is also found in batteries. It is also an important component in the control rods of nuclear power plants due to its ready nature in absorbing neutrons during a nuclear-fission process.
Mercury
The only transition metal to stay in a liquid form at room temperature, mercury is highly useful. The most well-known medical use of the element is in the manufacture of thermometers, and also in other measurement devices such as barometers, hydrometers, and pyrometers. It is used to remove gold and silver from amalgams, and is also a part of many electric switches and relays. Also, because it loses its resistance to electricity at very low temperatures, it is a very good candidate for superconductor research.
Titanium, Zirconium and Hafnium
Titanium is the 9th most abundant element available on earth, and the 2nd most common transition metal. Titanium is one of the strongest and lightest metals known to man. Due to its high tensile strength, it is used in the construction of fighter airplanes, racing cars, spacecraft, and other vehicles that face strong forces of pressure and mechanical stress on a regular basis. Titanium is also used to create artificial limbs and joints for people with physical disabilities. The metal is used to make pipes, that are part of nuclear power stations. Its compounds are also used in the production of white paint all over the world.
Zirconium and hafnium are always found together. While zirconium is used for making superconducting magnets, nuclear power plants, furnace linings, and various lotions; hafnium is used in light bulb filaments and various electrodes.
Chromium and Tungsten
Around 96% of the world’s chromium comes from parts of the old USSR, southern Africa, and the Philippines. It is commonly used in the process of electroplating for protective and decorative purposes. Besides this, due to its low ductility at low temperatures, it is also used to create non-ferrous alloys. It is also used for strengthening steel and manufacturing chrome/ tinted glass.
Tungsten is also used to strengthen steel and make it heat resistant, mainly because it is the metal with the highest melting point. This property also makes it important in light bulbs, missiles, and furnaces.
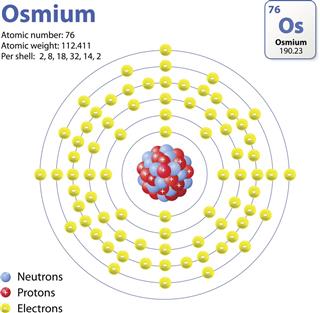
Iridium and Osmium
Both elements were discovered as by-products when platinum was first extracted from its ores. Iridium is mainly used to manufacture high-temperature laser crystals, and a platinum-iridium bar is used as the standard measure of a kilogram.
Osmium, on the other hand, is a hard metal that is resistant to wear and tear. Before tungsten, osmium was used as the filament for light bulbs. Nowadays, it is used in electric devices, phonograph needles, and the tips of fountain pens.
Palladium and Rhodium
Around the same time as the discovery of osmium, two more transition metal elements were found. Palladium is primarily used in the jewelry industry, as it resists tarnishing very well. It also forms white gold when alloyed with yellow gold. This element is used in the extraction of hydrogen as it can absorb the element up to 900 times of its own volume.
Rhodium is used to harden platinum, and is also important to improve optical instruments through the process of electroplating.
Niobium and Tantalum
Niobium is a element which is similar to steel in appearance. Upon polishing, it looks more like platinum. This transition metal is completely hypoallergenic, making it ideal for the creation of electronic devices, alloys, and jewelry.
Tantalum is relatively much rarer than most elements. It is highly resistant to chemical corrosion. Hence, it is used in capacitors and surgical instruments.
Some of these transition metals are also used in manufacturing stained glass, and chemical applications such as purifying crude oil. Research has indicated that these metals are going to be an important part in the cure of diseases such as cancer and AIDS. Apart from this, transition metals have a massive role in the living world. It is easy to say that without them, man would still be stranded in the stone age. In fact, due to their presence in our bodies, life would have been impossible without these elements. Due to this importance, the demand for them will never cease as long as life exists on Earth.